MLE-12 Cell Line: A Key Model for Pulmonary Research You Need to Know
Introduction: What are MLE-12 Cells?
In the quest to understand the complex physiological and pathological mechanisms of the lungs, stable and representative in vitro cell models play a crucial role. The MLE-12 cell line is one such widely adopted tool. It originated from the lung tissue of adult FVB/N mice and was established through an immortalization process mediated by the Simian Virus 40 (SV40) Large T-antigen [1]. MLE-12 cells are extensively recognized as a representative cell line for mimicking the functions of pulmonary Alveolar Type II (ATII) cells. ATII cells are pivotal in maintaining alveolar homeostasis, producing and secreting surfactant, and contributing to lung repair and regeneration following injury. Consequently, MLE-12 cells, capable of effectively simulating these functions, provides researchers with a convenient and reproducible platform to delve into lung biology, disease pathogenesis, and potential therapeutic strategies.
Main Biological Characteristics of MLE-12 Cells
Understanding the biological characteristics of MLE-12 cells is fundamental to their effective utilization as a research model.
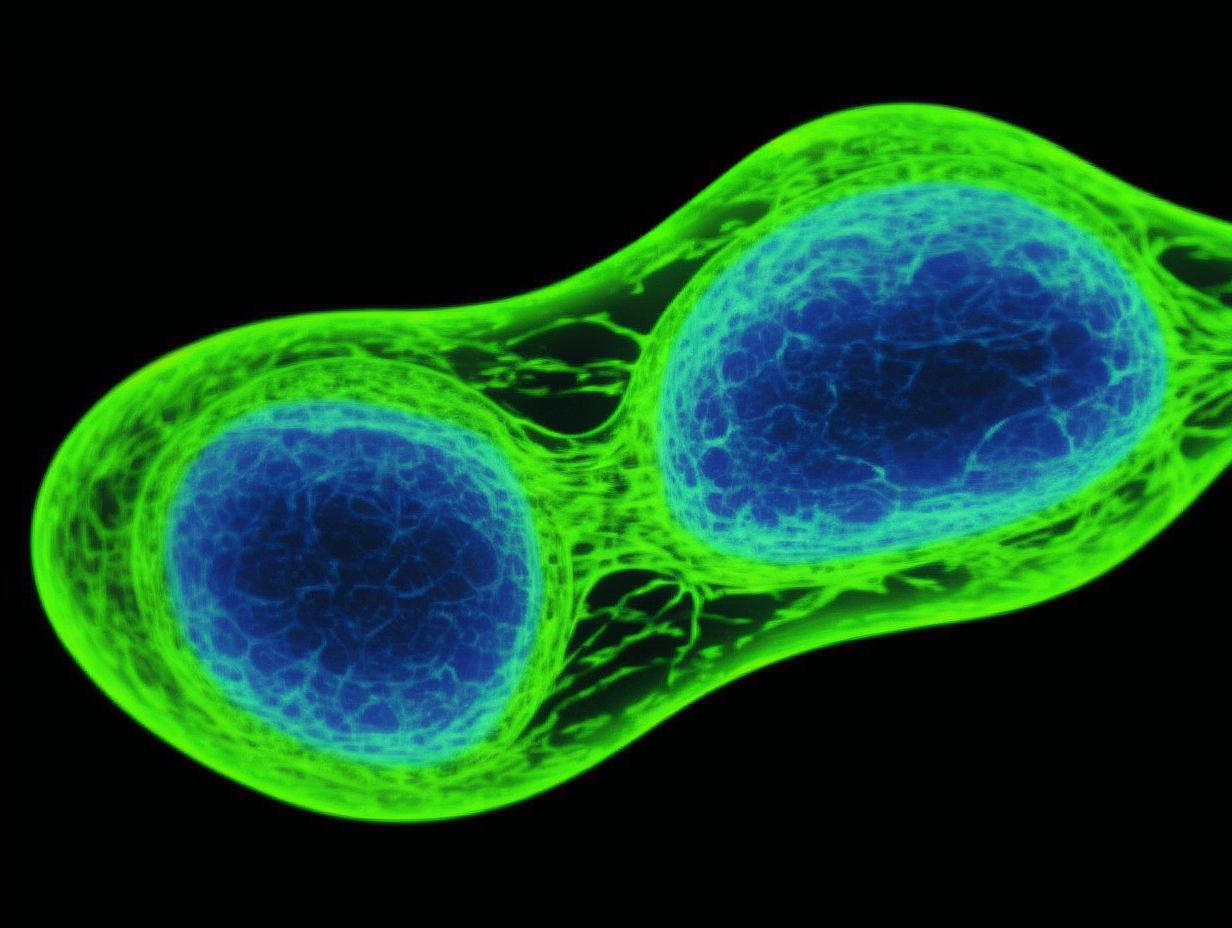
Morphological Features:
In vitro, MLE-12 cells typically exhibit a classic epithelial-like morphology, appearing polygonal or cobblestone-shaped, and are anchorage-dependent for growth. They can form tight cell monolayers, which is particularly important for studying alveolar epithelial barrier function. Microscopically, they present with relatively large nuclei and distinct cytoplasm.
Key Molecular Markers:
As ATII-like cells, MLE-12 cells express several molecular markers characteristic of type II cells. Most notably, these include Surfactant Proteins (SPs), such as SP-A, SP-B, and SP-C. These proteins are essential for reducing alveolar surface tension and preventing alveolar collapse. It is important to note that while MLE-12 cells express these markers, their expression levels and patterns may not entirely mirror those of primary ATII cells, primarily due to phenotypic alterations that can occur during immortalization and long-term in vitro culture [2]. Additionally, they express common epithelial markers like cytokeratins. The expression of the SV40 Large T-antigen serves as a hallmark of their immortalization.
Proliferation Properties and General Culture Conditions:
MLE-12 cells possess a robust proliferative capacity and can be stably passaged under appropriate culture conditions. Typically, they thrive in DMEM/F12 or similar mixed media supplemented with fetal bovine serum (FBS, commonly at 2-10%). The standard culture environment requires a humidified incubator at 37°C with 5% CO2. Due to their relatively rapid proliferation, regular subculturing is necessary to maintain cell health and an appropriate cell density, avoiding alterations in cell characteristics due to overconfluence.
Accelerate pulmonary drug discovery with our MLE-12 cells. This effective in vitro model helps evaluate therapeutic impacts and toxicity on lung cells. Order now>>
Advantages of MLE-12 Cells as a Research Model
The widespread application of MLE-12 cells in basic and translational pulmonary research stems from their numerous advantages.
1. Ease of Culture and Maintenance, and High Cell Yield:
Compared to primary ATII cells, which are difficult to isolate and purify, require demanding culture conditions, and have a limited lifespan, the MLE-12 cell line is readily accessible and easy to culture. They can be passaged multiple times, providing an abundant and consistent source of cells for experiments. This is particularly beneficial for studies requiring large cell numbers, such as high-throughput drug screening.
2. Greater Uniformity and Reproducibility Compared to Primary Cells:
Immortalized cell lines generally offer higher inter-batch and inter-experiment uniformity than primary cells. This means that experiments conducted using MLE-12 cells tend to yield more reproducible results, reducing variability introduced by donor differences or isolation procedures, thereby facilitating more reliable conclusions.
3. Applicability in Simulating Certain Pulmonary Physiological and Pathological Processes:
Despite their limitations, MLE-12 cells hold significant value in mimicking specific pulmonary physiological functions (e.g., aspects of surfactant metabolism) and pathological processes. For instance, they are commonly employed to study:
a. Lung Injury and Repair: Cellular responses to oxidative stress (e.g., H2O2), inflammatory stimuli (e.g., LPS, TNF-α), viral or bacterial infections, and physicochemical injuries (e.g., bleomycin, silica), including cell survival, apoptosis, inflammatory mediator release, and changes in barrier function [3].
b. Drug Screening and Toxicological Evaluation: As an in vitro platform for assessing the effects of potential therapeutic agents on lung epithelial cells or evaluating the cytotoxicity of inhaled particulate matter and environmental pollutants.
c. Signal Transduction Pathway Research: Investigating the activation and regulation of specific signaling pathways (e.g., TGF-β, Wnt, NF-κB) relevant to alveolar cell function and injury repair.
Main Limitations of MLE-12 Cells
To scientifically and objectively interpret experimental results based on MLE-12 cells, it is imperative to fully acknowledge their inherent limitations.
Differences Between Immortalized and Primary Cells:
The immortalization process itself (e.g., introduction of SV40 T-antigen) can alter the gene expression profile and biological behavior of cells, causing them to differ from primary ATII cells in vivo. For example, cell cycle regulation and signal transduction pathways may be altered, and certain differentiation characteristics might be diminished or lost.
Discrepancies Between In Vitro Models and In Vivo Environment:
In vitro monolayer cell culture cannot fully replicate the complex pulmonary microenvironment found in vivo. In the body, alveolar epithelial cells interact closely with various cell types, including fibroblasts, endothelial cells, and immune cells, as well as the extracellular matrix. This intricate intercellular communication and three-dimensional architecture are absent in traditional 2D cultures, potentially affecting cellular responses to stimuli and drug metabolism.
Potentially Inconsistent Gene Expression Profiles with In Vivo Type II Cells:
As previously mentioned, although MLE-12 cells express key markers of type II cells, their abundance, subcellular localization, or post-translational modifications may differ from the in vivo situation. Genes highly expressed in primary cells might be downregulated in MLE-12 cells, and vice versa. Therefore, any critical findings should, whenever possible, be validated in primary cells or animal models.
Effectively model lung pathologies with our MLE-12 cells. Ideal for investigating mechanisms of fibrosis, inflammation, and other pulmonary conditions. Learn more>>
Conclusion: Understanding and Utilizing MLE-12 Cells Correctly
The MLE-12 cell line is undoubtedly a powerful and convenient tool in the field of pulmonary biology and disease research. Its advantages, including ease of culture, good homogeneity, and an ability to model various pulmonary physiological and pathological processes, have significantly advanced our understanding of alveolar epithelial cell function, lung injury and repair mechanisms, and drug targets.
However, researchers must be acutely aware of the inherent limitations of MLE-12 cells as an immortalized in vitro model. Any experimental results derived from MLE-12 cells should be interpreted with caution before extrapolation to more complex physiological systems (such as animal models or humans) and, where feasible, validated using complementary research models (e.g., primary cells, organoid models, animal models). Only by doing so can we maximize the value of MLE-12 cells and make solid contributions towards ultimately conquering pulmonary diseases.
References:
1.Wikenheiser KA, Vorbroker DK, Rice WR, Clark JC, Bachurski CJ, Oie HK, Whitsett JA. Production of immortalized distal respiratory epithelial cell lines from surfactant protein C/simian virus 40 large tumor antigen transgenic mice. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4171-5. Erratum in: Proc Natl Acad Sci U S A 1993 Aug 15;90(16):7828.
2.Kosmider B, Lin CR, Karim L, Tomar D, Madesh M, Bahmed K, Kelsen SG, Criner GJ. Surfactant protein C and B processing in type II alveolar epithelial cells from normal and individuals with surfactant protein C G100S mutation. Am J Physiol Lung Cell Mol Physiol. 2018 Feb 1;314(2):L217-L231.
3.Roque W, Romero F. MLE-12 cells as a model for in vitro studies of alveolar type II epithelium. Cell Biol Int. 2000;24(10):667-75.

